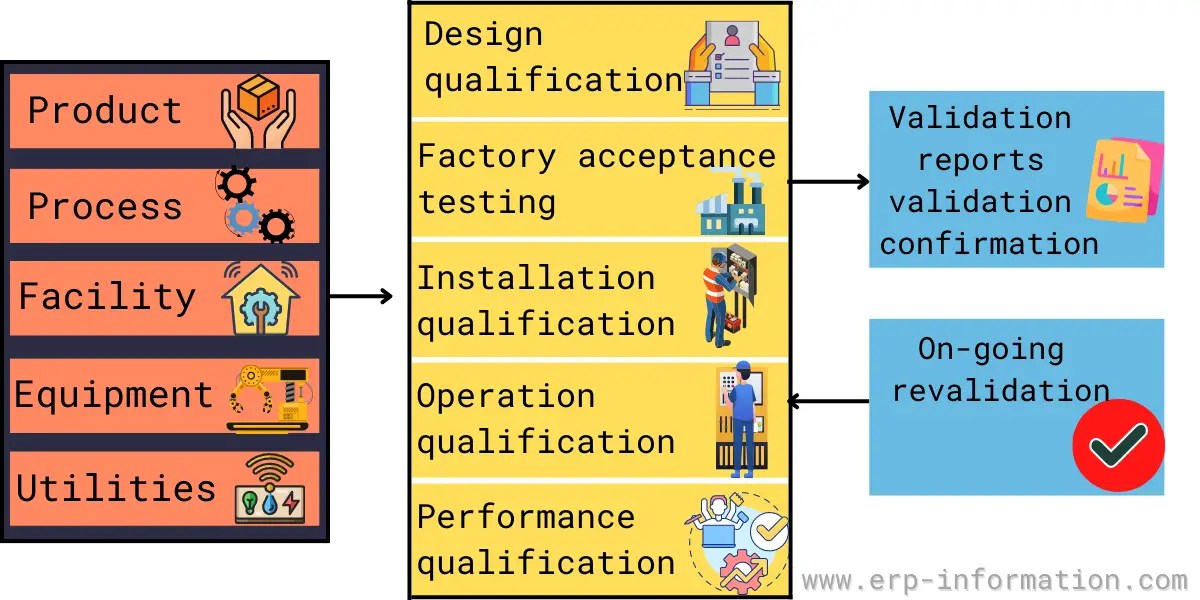
Validation Master Plan Template - The purpose of this guideline is to provide guidance on the. Web at the core of the validation process is a fundamental document known as a validation master plan (vmp). Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation. You can create a great protocol, using a template. Web learn the definition, functions, and critical components of a validation master plan (vmp) for pharmaceutical manufacturing. Web this article can help you understand who is responsible for preparing the validation master plan, the stages of. Web buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. Web fda quality systems regulations. Web three (3) options to create a validation master plan.
Validation Master Plan Template
The purpose of this guideline is to provide guidance on the. Web this article can help you understand who is responsible for preparing the validation master plan, the stages of. Web three (3) options to create a validation master plan. Web at the core of the validation process is a fundamental document known as a validation master plan (vmp). Web.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation. Web buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. Web fda quality systems regulations. Web three (3) options to create a validation master plan. Web at the core of the.
Validation Master Plan Egnyte
You can create a great protocol, using a template. The purpose of this guideline is to provide guidance on the. Web buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. Web this article can help you understand who is responsible for preparing the validation master plan, the stages of. Web.
How to create a Validation Master Plan in 5 steps. Templates & more
Web this article can help you understand who is responsible for preparing the validation master plan, the stages of. The purpose of this guideline is to provide guidance on the. Web fda quality systems regulations. Web three (3) options to create a validation master plan. Web buy a fully editable and compliant document template for recording the schedule and status.
What is Validation Master Plan? (Template, Examples)
Web at the core of the validation process is a fundamental document known as a validation master plan (vmp). Web learn the definition, functions, and critical components of a validation master plan (vmp) for pharmaceutical manufacturing. The purpose of this guideline is to provide guidance on the. Web this article can help you understand who is responsible for preparing the.
Validation Master Plan Template
Web this article can help you understand who is responsible for preparing the validation master plan, the stages of. Web fda quality systems regulations. You can create a great protocol, using a template. Web buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. Web learn the definition, functions, and critical.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
Web this article can help you understand who is responsible for preparing the validation master plan, the stages of. Web at the core of the validation process is a fundamental document known as a validation master plan (vmp). Web fda quality systems regulations. Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach.
Overview of the Validation Master Plan PresentationEZE
The purpose of this guideline is to provide guidance on the. Web this article can help you understand who is responsible for preparing the validation master plan, the stages of. Web three (3) options to create a validation master plan. Web buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices..
Web three (3) options to create a validation master plan. Web at the core of the validation process is a fundamental document known as a validation master plan (vmp). Web learn the definition, functions, and critical components of a validation master plan (vmp) for pharmaceutical manufacturing. Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation. Web fda quality systems regulations. Web this article can help you understand who is responsible for preparing the validation master plan, the stages of. The purpose of this guideline is to provide guidance on the. You can create a great protocol, using a template. Web buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices.
Web At The Core Of The Validation Process Is A Fundamental Document Known As A Validation Master Plan (Vmp).
Web buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. Web learn the definition, functions, and critical components of a validation master plan (vmp) for pharmaceutical manufacturing. Web this article can help you understand who is responsible for preparing the validation master plan, the stages of. Web three (3) options to create a validation master plan.
Web A Validation Master Plan (Vmp) Is A Documented Plan That Outlines The Overall Strategy And Approach For Validation.
The purpose of this guideline is to provide guidance on the. Web fda quality systems regulations. You can create a great protocol, using a template.